Enterprise Document Management Software USA – Qualityze
In the digital age, U.S. companies must manage an ever-growing volume of documents while ensuring compliance with industry standards and regulatory requirements. From FDA regulations to ISO documentation guidelines, businesses need a secure and efficient way to manage, control, and track documents across the organization. This is where Enterprise Document Management Software USA comes into play, and Qualityze provides one of the most trusted, cloud-based solutions to meet these needs.
Why US Companies Need Enterprise Document Management Software
For enterprises in the USA, managing documents manually or through disconnected systems creates risks like version errors, compliance issues, and lost data. A robust Enterprise Document Management Software helps to:
Maintain centralized, secure document storage
Ensure version control and access management
Support FDA, ISO, and other regulatory compliance
Enable quick search and retrieval of documents
Automate approval workflows
Keep audit-ready records
Qualityze: Leading Enterprise Document Management Software in USA
Qualityze Enterprise Document Management Software is a cloud-based solution built on the Salesforce platform. It provides enterprises with powerful tools to manage documents throughout their lifecycle—creation, review, approval, distribution, and archival—while ensuring compliance and security.
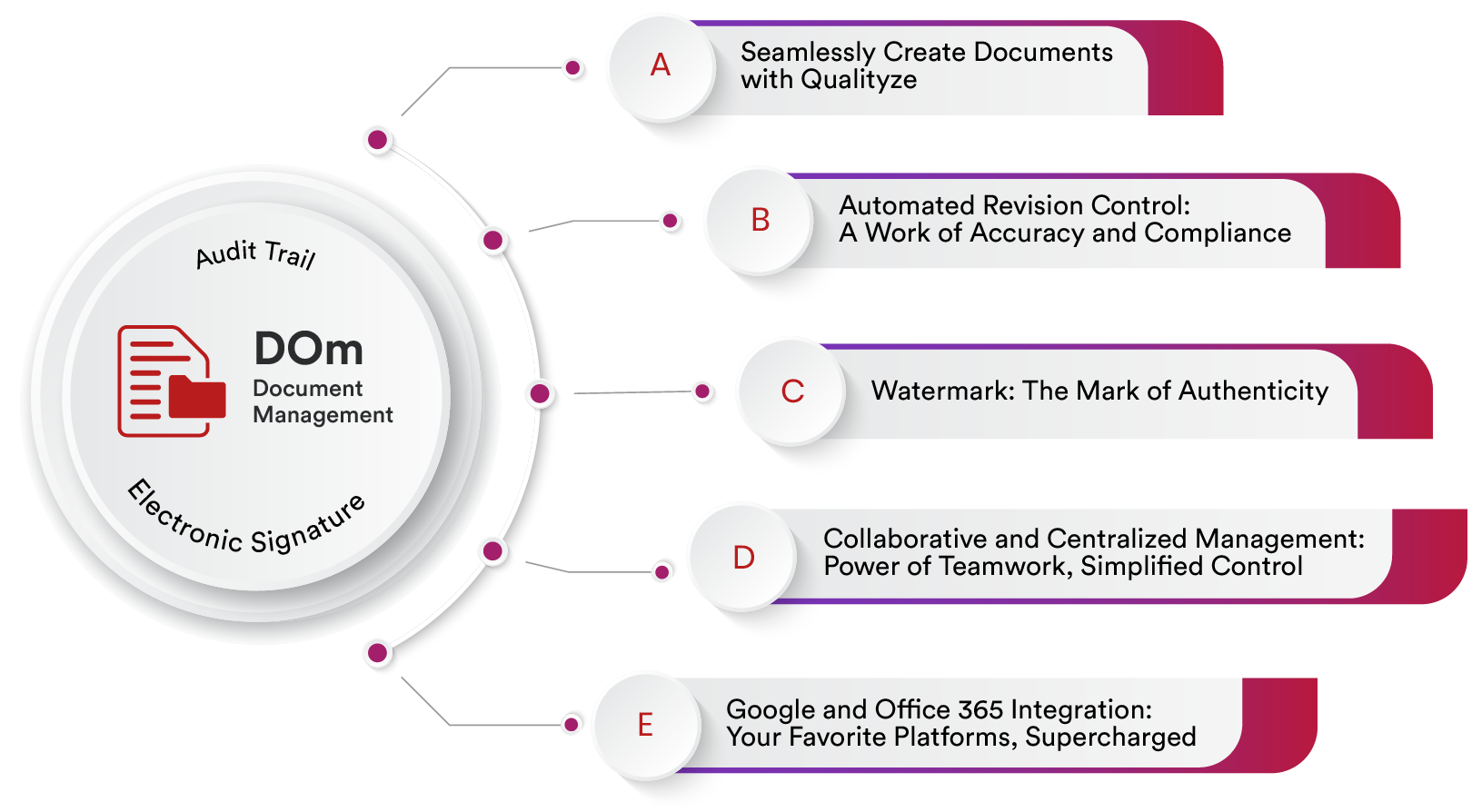
Key Features of Qualityze Document Management Software
Centralized Repository – Store and manage documents in one secure platform.
Version Control – Track document revisions and maintain accuracy.
Automated Workflows – Streamline approvals, reviews, and updates.
Audit Trails – Maintain complete records for compliance audits.
Role-Based Access – Ensure secure access to sensitive documents.
Integration Capabilities – Seamlessly connect with other enterprise systems.
Benefits for US Enterprises
Regulatory Compliance – Supports FDA 21 CFR Part 11, ISO, and other U.S. regulations.
Improved Collaboration – Teams can access and collaborate on documents anytime, anywhere.
Enhanced Productivity – Reduces manual errors and accelerates approval cycles.
Cloud-Based Flexibility – Scalable and accessible solution for growing organizations.
Secure & Reliable – Built on Salesforce for enterprise-grade security and reliability.
Why Choose Qualityze in the USA?
Choosing Enterprise Document Management Software USA is not just about storing files—it’s about ensuring compliance, maintaining accuracy, and driving efficiency. Qualityze stands out with its intuitive design, configurable workflows, and ability to adapt to the specific compliance needs of U.S. industries such as life sciences, manufacturing, food & beverages, automotive, and more.
Conclusion
For enterprises seeking a reliable, compliant, and cloud-based Enterprise Document Management Software USA, Qualityze offers the perfect solution. With robust features, Salesforce-powered security, and regulatory alignment, Qualityze helps U.S. businesses manage documents effectively, streamline compliance, and achieve operational excellence. Read more - https://www.qualityze.com/document-management
In the digital age, U.S. companies must manage an ever-growing volume of documents while ensuring compliance with industry standards and regulatory requirements. From FDA regulations to ISO documentation guidelines, businesses need a secure and efficient way to manage, control, and track documents across the organization. This is where Enterprise Document Management Software USA comes into play, and Qualityze provides one of the most trusted, cloud-based solutions to meet these needs.
Why US Companies Need Enterprise Document Management Software
For enterprises in the USA, managing documents manually or through disconnected systems creates risks like version errors, compliance issues, and lost data. A robust Enterprise Document Management Software helps to:
Maintain centralized, secure document storage
Ensure version control and access management
Support FDA, ISO, and other regulatory compliance
Enable quick search and retrieval of documents
Automate approval workflows
Keep audit-ready records
Qualityze: Leading Enterprise Document Management Software in USA
Qualityze Enterprise Document Management Software is a cloud-based solution built on the Salesforce platform. It provides enterprises with powerful tools to manage documents throughout their lifecycle—creation, review, approval, distribution, and archival—while ensuring compliance and security.
Key Features of Qualityze Document Management Software
Centralized Repository – Store and manage documents in one secure platform.
Version Control – Track document revisions and maintain accuracy.
Automated Workflows – Streamline approvals, reviews, and updates.
Audit Trails – Maintain complete records for compliance audits.
Role-Based Access – Ensure secure access to sensitive documents.
Integration Capabilities – Seamlessly connect with other enterprise systems.
Benefits for US Enterprises
Regulatory Compliance – Supports FDA 21 CFR Part 11, ISO, and other U.S. regulations.
Improved Collaboration – Teams can access and collaborate on documents anytime, anywhere.
Enhanced Productivity – Reduces manual errors and accelerates approval cycles.
Cloud-Based Flexibility – Scalable and accessible solution for growing organizations.
Secure & Reliable – Built on Salesforce for enterprise-grade security and reliability.
Why Choose Qualityze in the USA?
Choosing Enterprise Document Management Software USA is not just about storing files—it’s about ensuring compliance, maintaining accuracy, and driving efficiency. Qualityze stands out with its intuitive design, configurable workflows, and ability to adapt to the specific compliance needs of U.S. industries such as life sciences, manufacturing, food & beverages, automotive, and more.
Conclusion
For enterprises seeking a reliable, compliant, and cloud-based Enterprise Document Management Software USA, Qualityze offers the perfect solution. With robust features, Salesforce-powered security, and regulatory alignment, Qualityze helps U.S. businesses manage documents effectively, streamline compliance, and achieve operational excellence. Read more - https://www.qualityze.com/document-management
Enterprise Document Management Software USA – Qualityze
In the digital age, U.S. companies must manage an ever-growing volume of documents while ensuring compliance with industry standards and regulatory requirements. From FDA regulations to ISO documentation guidelines, businesses need a secure and efficient way to manage, control, and track documents across the organization. This is where Enterprise Document Management Software USA comes into play, and Qualityze provides one of the most trusted, cloud-based solutions to meet these needs.
Why US Companies Need Enterprise Document Management Software
For enterprises in the USA, managing documents manually or through disconnected systems creates risks like version errors, compliance issues, and lost data. A robust Enterprise Document Management Software helps to:
Maintain centralized, secure document storage
Ensure version control and access management
Support FDA, ISO, and other regulatory compliance
Enable quick search and retrieval of documents
Automate approval workflows
Keep audit-ready records
Qualityze: Leading Enterprise Document Management Software in USA
Qualityze Enterprise Document Management Software is a cloud-based solution built on the Salesforce platform. It provides enterprises with powerful tools to manage documents throughout their lifecycle—creation, review, approval, distribution, and archival—while ensuring compliance and security.
Key Features of Qualityze Document Management Software
Centralized Repository – Store and manage documents in one secure platform.
Version Control – Track document revisions and maintain accuracy.
Automated Workflows – Streamline approvals, reviews, and updates.
Audit Trails – Maintain complete records for compliance audits.
Role-Based Access – Ensure secure access to sensitive documents.
Integration Capabilities – Seamlessly connect with other enterprise systems.
Benefits for US Enterprises
Regulatory Compliance – Supports FDA 21 CFR Part 11, ISO, and other U.S. regulations.
Improved Collaboration – Teams can access and collaborate on documents anytime, anywhere.
Enhanced Productivity – Reduces manual errors and accelerates approval cycles.
Cloud-Based Flexibility – Scalable and accessible solution for growing organizations.
Secure & Reliable – Built on Salesforce for enterprise-grade security and reliability.
Why Choose Qualityze in the USA?
Choosing Enterprise Document Management Software USA is not just about storing files—it’s about ensuring compliance, maintaining accuracy, and driving efficiency. Qualityze stands out with its intuitive design, configurable workflows, and ability to adapt to the specific compliance needs of U.S. industries such as life sciences, manufacturing, food & beverages, automotive, and more.
Conclusion
For enterprises seeking a reliable, compliant, and cloud-based Enterprise Document Management Software USA, Qualityze offers the perfect solution. With robust features, Salesforce-powered security, and regulatory alignment, Qualityze helps U.S. businesses manage documents effectively, streamline compliance, and achieve operational excellence. Read more - https://www.qualityze.com/document-management
0 Reacties
0 aandelen
1K Views
0 voorbeeld